Cosmetic Testing
Animal testing for cosmetic products has a long history, with multiple investigations over the years revealing the suffering of animals in labratories across the world. While much progress has been made to end this practice, animals still suffer from cruel, painful testing for the development of makeup, perfume, soaps and other cosmetics.

2020 Cosmetic Testing Ban

In 2020, Australia banned the use of new animal test data for cosmetics, as set out in the Industrial Chemicals Act 2019, which commenced on 1 July 2020. The Commonwealth Department of Health managed the implementation of this ban, with the assistance of the National Health and Medical Research Council (NHMRC).
Despite this, cosmetics sold in Australia can still be developed using data from animal testing due to a number of loopholes. While the law means that ingredients used purely for cosmetics and finished cosmetics products can't be tested on animals, chemicals that are intended for use in cosmetics may still be tested on animals provided the purpose for the testing is justified by a non-cosmetic purpose.
For example, a chemical ingredient intended to be used in a lipstick product (cosmetic) and a clothes detergent (non-cosmetic), may be tested on animals provided the testing meets the requirements of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
This also applies to imported cosmetics. Under the Industrial Chemicals Act 2019, animal testing data may still be used to support the introduction of new chemical ingredients for use in cosmetics provided the chemical will also be used in a non-cosmetic product.
Additionally, some companies will coducts offshore animal testing, in order to develop products which can be sold in markets with more stringent requirements for animal testing. Until 2021, cosmetic testing was mandatory for all cosmetic products sold in China. In 2021, regulations changed, meaning that products free from testing could be sold, however 'special cosmetics,' including hair dye, sunscreen and products for children, still required animal testing. This means that some companies, such as Nivea, will still conduct animal testing outside of Australia, even while selling products in the country.
Below is a summary of the most common tests performed in cosmetic testing; the Draize Eye test and the Skin Irritation tests which were developed in the 1940s and the Lethal Dose Test, which was originally developed in 1927.
In most cases of cosmetic testing, the animals-usually albino rabbits, are bred for the lab in factory farms. They are then sold to labs, where they are often housed in isolation, in bare, wire cages without sufficient space to move or environmental enrichment.
Common Cosmetic Testing Procedures
Draize Test
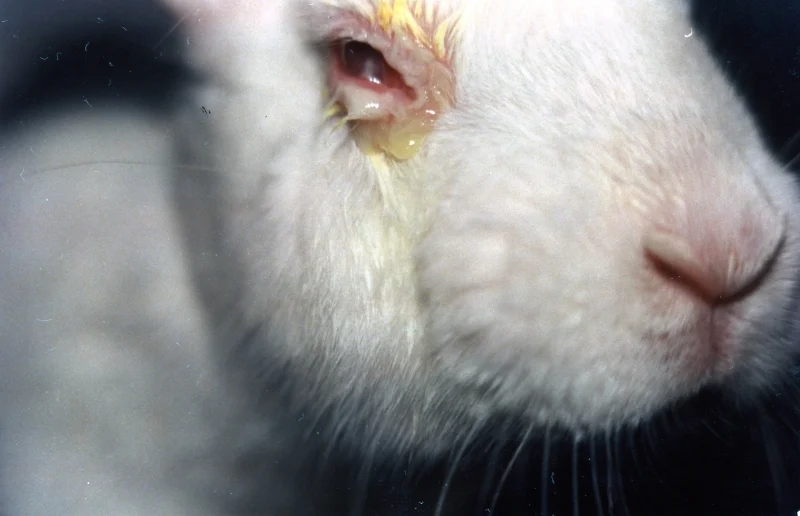
The Draize test involves applying an amount of the substance under study to an animal's eye or skin for several hours, and then observing whether or not irritation occurs over the following week or two. Test animals are then put to death in a brutal manner of cervical dislocation after the sometimes maiming and often painful test.
These tests cause extreme discomfort and pain to the animals involved. Rabbits are very fragile animals and usually live in groups. Under stress they seek comfort in one another. This is however not permitted in labs where they are all individually isolated for testing.
In the eye version of the Draize test, rabbits are placed in restraining stocks and their eyelids are held open with clips-in some cases for days at a time-to keep them from blinking away the test solutions.
Rabbits have no tear ducts so, unlike humans, they can't cry out harmful substances from their eye. This means that in the Draize eye test the rabbit's eye is exposed to more of the test chemical for longer periods, which is one of the main reasons why rabbits are chosen for this procedure.
Skin Irritation Test
The skin irritation test involves shaving a part of the rabbit's body and applying a test substance to their skin. The site is covered with a gauze patch for up to four hours, after which the patch is removed and the remaining substance wiped away. A wound is allowed to develop at the site for up to 14 days, and the degree of skin damage is then assessed. A chemical is considered to be an irritant if it causes reversible skin lesions, such as inflammation, that heal partially or totally by the end of the observation period.
The test may cause rabbits to suffer from inflamed skin, ulcers, bleeding, and bloody scabs. There is no requirement that animals be provided with pain-relieving drugs during this prolonged process.
Animal-based skin irritation studies have never been properly validated.
Lethal Dose Test
Finally, the lethal dose test, or lethal dose 50 (LD50) is a test where animals are given increasing doses of a substance until half of them die. This informs scientists of a substance's 'lethal dose'.
Symptoms of lethal substance doseage include tremors, convulsions, swellings, bleeding from the mouth and nose, bulging eyeballs and difficulty breathing.


